Abstract
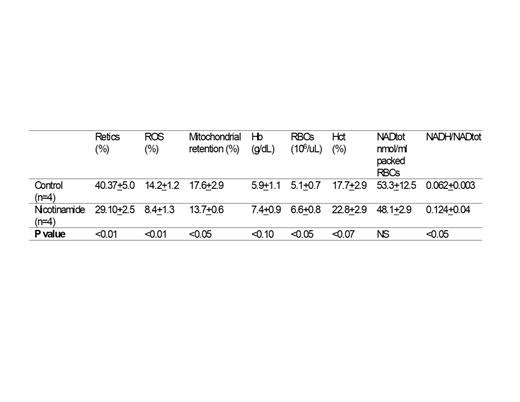
The polymerization of deoxygenated HbS molecules in the red blood cells (RBCs) of patients with sickle cell disease (SCD) causes RBC destruction resulting in chronic pain, debilitating acute pain crises, strokes, multi-organ damage and a reduced life span. Therapeutic options remain limited. Bone marrow transplantation can be curative but is not an option for the majority of patients. Gene therapy interventions that also offer the promise of a cure are under investigation but are not likely to be available to the vast majority of patients in the near future. Hydroxyurea, the first drug approved for treatment of SCD, increases levels of Fetal Hemoglobin (HbF) that inhibit polymerization of HbS molecules but is not effective in all patients while a more powerful HbF-inducing drug, the DNA methyltransferase inhibitor decitabine has yet to be approved. L-glutamine, another approved therapeutic option, increases NAD redox potential and decreases reactive oxygen species (ROS) in the sickle RBCs to reduce symptoms. In our laboratory we have observed that increased ROS is associated with the retention of mitochondria in the SCD RBCs and have hypothesized that the abnormal presence of mitochondria in these cells is a major source of ROS (Jagadeeswaran et al Exp Hematol 50:46-52, 2017). In this investigation we have tested the hypothesis that chronic oral supplementation with nicotinamide, a direct precursor of NAD synthesis, would improve NAD redox potential, decrease mitochondrial retention and ROS in SCD RBCs, and reduce anemia in the SCD mouse model. The effect of nicotinamide was tested in SCD mice whose drinking water was supplemented for three months with 1% nicotinamide. The percentage of RBCs retaining mitochondria and the levels of ROS were determined by flow cytometric assays using the mitochondrial-specific dye TMRM and the ROS probe CM-H2DCFDA, respectively. In SCD mice receiving nicotinamide the fraction of RBCs retaining mitochondria was reduced 22.1% (p<0.05) and the level of ROS in RBCs was reduced 41% (p<0.01) compared to control SCD mice. The reticulocyte percentage was reduced 28% in nicotinamide-treated SCD mice compared to control SCD mice (p<0.01). The total RBC count was 30% higher (p<0.05) in nicotinamide-treated mice (6.61±0.76 X 10 6/μl) compared to control SCD mice (5.08±0.70 X 10 6/μl). Similar differences in hematocrit and total hemoglobin were also observed but failed to reach statistical significance. Total NAD levels were not significantly different in SCD mice receiving nicotinamide compared to control SCD mice (p<0.05), but the NADH/NAD total ratio was increased 2 fold (p<0.05). These results show that oral administration of high doses of nicotinamide decreases mitochondrial retention and ROS in SCD RBCs and improves NAD redox potential and anemia in SCD mice. These effects strongly suggest that additional studies be performed to investigate nicotinamide as a therapeutic option in SCD.
Saunthararajah: EpiDestiny: Consultancy, Current holder of individual stocks in a privately-held company, Membership on an entity's Board of Directors or advisory committees, Patents & Royalties.